Thermodynamics
|
4A30.10 |
THERMAL PROPERTIES OF MATTER |
|
|
Solid Expansion |
||
Bimetallic Strip |
||
|
|
||
|
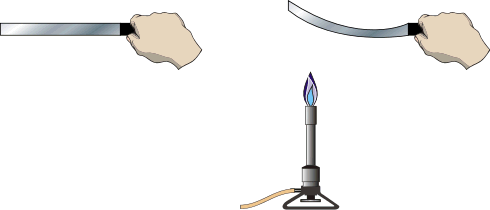
A bimetal strip is brass on one side and steel on the other. When heated over a Bunsen burner, the strip curves toward the steel side. OR, when cooled in liquid nitrogen the strip curves toward the brass side. |
||
|
4A30.20 |
THERMAL PROPERTIES OF MATTER |
|
|
Solid Expansion |
||
Ball and Ring |
||
|
Disc 14-11 |
||
|
Try putting the ring around the ball. At room temperature the ball is slightly larger the ring and will not pass through the ring Heat the ring over the Bunsen burner and try again. You may also cool the ball in liquid nitrogen. This takes several minutes.
|
||
|
4A40.30 |
THERMAL PROPERTIES OF MATTER |
|
|
Properties of Materials at Low Temperatures |
||
Smashing Rose and Tube |
||
|
|
||
|
Pour LN2 into the beaker. Put the end of the rubber hose into the nitrogen. When the bubbling subsides, take it out and break it with a hammer. Put the flower or some lettuce into the liquid nitrogen. When frozen, crumble it with your hand.
|
||
|
4B20.10 |
HEAT AND THE FIRST LAW |
|
|
Convection |
||
Convection Tube |
||
|
|
||
|
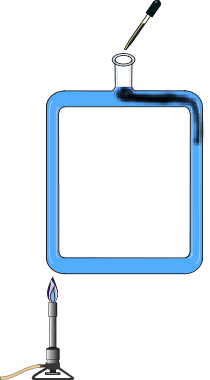
A square tube is filled with water. Light the Bunsen burner and place under one side. Using the eye dropper, drop several drops of ink into the top hole. The ink shows the direction of fluid flow. Move the burner to the other side of the tube to reverse the flow.
|
||
|
4B30.21 |
HEAT AND THE FIRST LAW |
|
|
Conduction |
||
Conduction Rods |
||
|
|
||
|
Four rods with liquid crystal thermometers are placed in hot water to show different conduction rates.
|
||
|
4B40.10 |
HEAT AND THE FIRST LAW |
|
|
Radiation |
||
Light the Match |
||
|
Disc 22-04 |
||
|
Two reflectors are set at opposite ends of the lecture bench. One contains a heater controlled by a variac. The other has a match at the focal point of the reflector. Turn the variac all the way up and wait. The match will light in about 1 minute. If it takes longer, something is wrong. Alignment is critical! |
||
|
4B50.25 |
HEAT AND THE FIRST LAW |
|
|
Heat Transfer Applications |
||
Heating a Water Balloon |
||
|
|
||
|
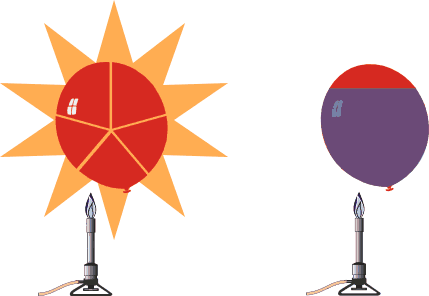
A balloon filled with water will not pop like a similar balloon filled with air. |
||
|
4B60.10 |
HEAT AND THE FIRST LAW |
|
|
Mechanical Equivalent of Heat |
||
Dropping Lead Shot |
||
|
Disc 15-02 |
||
|
Stick the temperature probe (not thermometer!) into the bag of lead shot to find its initial temperature. Drop the bag from the height of 2 meters 10-20 times. Take the temperature of the lead again. The temp should rise 2-3 degrees Celsius. The specific heat of lead is 0.031 cal/(g C). The disc shows inverting a tube 10 times. |
||
|
4B70.20 |
HEAT AND THE FIRST LAW |
|
|
Adiabatic Processes |
||
Expansion Cloud Chamber |
||
|
|
||
|
Pressurize a jug of saturated water vapor with and without smoke particles. When the pressure is released, a cloud will form in the jug with the smoke particles.
|
||
|
4C30.10 |
CHANGE OF STATE |
|
|
Phase Changes: Liquid-Gas |
||
Boiling by Cooling |
||
|
Disc 15-10 |
||
|
Heat water to boiling in a Remove heat, stopper and invert. Boiling continues again as ice is added to the dimple in the flask. |
||
|
4C31.30 |
CHANGE OF STATE |
|
|
Cooling by Evaporation |
||
Drinking Bird |
||
|
Disc 15-12 |
||
|
Soak the bird's head in the water and let him "drink". |
||
|
4D10.10 |
KINETIC THEORY |
|
|
Brownian Motion |
||
Brownian Motion Cell |
||
|
|
||
|
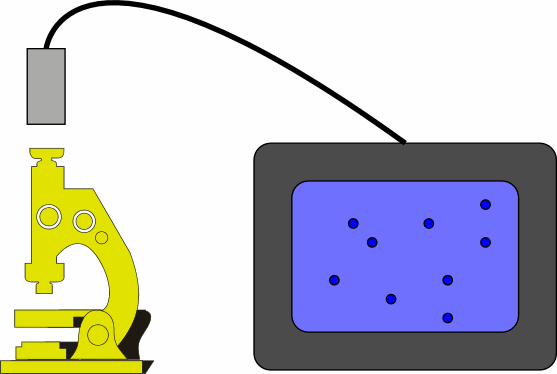
Observe the motion of particles in a smoke cell through a microscope.
|
||
|
4D20.10 |
KINETIC THEORY |
|
|
Mean Free Path |
||
Crookes' Radiometer |
||
|
Disc 14-23 |
||
|
This radiometer spins in the opposite direction from what theory predicts; the white side moves forward. Turn on the light and watch it spin. |
||
|
4D30.20 |
KINETIC THEORY |
|
|
Kinetic Motion |
||
Molecular Motion Demonstrator |
||
|
|
||
|
The kinetic theory of gas is expanded using a variable speed molecular motion demonstrator on the overhead. |
||
|
4E10.20 |
GAS LAW |
|
|
Constant Pressure |
||
Balloon in LN2 |
||
|
|
||
|
An air-filled balloon sits in a beaker. Pour liquid nitrogen over the balloon and watch it shrink. Take the balloon out and it blows back up. |
||
|
4E30.10 |
GAS LAW |
|
|
Constant Volume |
||
Constant Volume Bulb |
||
|
Disc 16-02 |
||
|
The gauge measures the pressure in the sealed sphere in pounds per square inch. Atmospheric pressure is 14.7 lbs per square inch. Read the pressure when the bulb is immersed in boiling water, ice water and LN2 (77 Kelvin). Different gases may be put into the bulb. |
||
|
4F30.10 |
ENTROPY AND THE SECOND LAW |
|
|
Heat Cycles |
||
|
||
|
Disc 15-06 |
||
|
The hot air chamber of the Light the burner. If the engine is cold, it takes several minutes before it is hot enough to run. Start the engine by turning the fly-wheel in the proper direction (see your instructions) |
||